Medical Device Academy has announced the upcoming release of its 2026 Lead Auditor Training Course for Medical Devices and Software as a Medical Device (SaMD). The on-demand, self-paced course will be available for pre-order on December 1, ahead of its official launch on December 19.
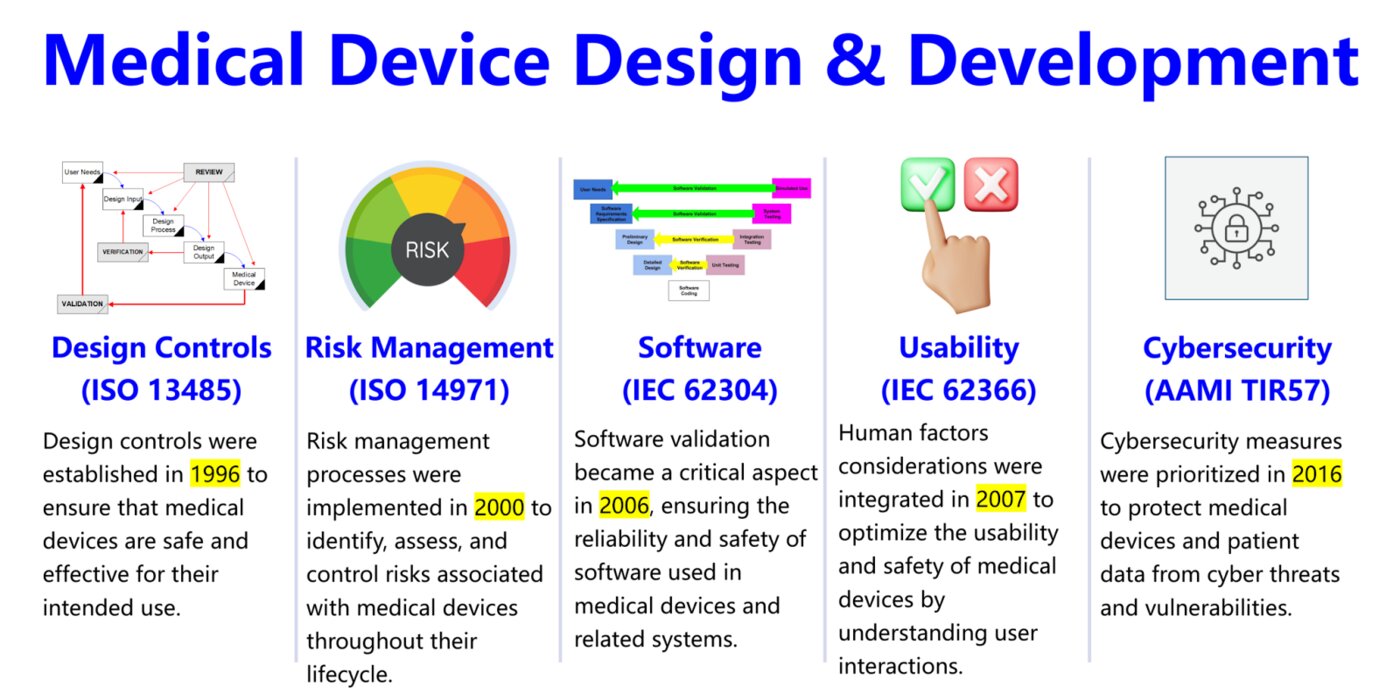
According to Rob Packard, founder and President of Medical Device Academy, the course was modernized to provide additional training in risk management, software, usability, and cybersecurity. “Medical device companies are audited every year, and those auditors need training on recently developed standards,” Packard said. “This course allows them to focus on the standards they don’t already know at their own pace.”
(Source: Medical Device Academy)
Packard, who has developed previous in-person auditor courses, explained that this new version moves beyond the traditional five-day, in-person format. “When you are trying to absorb complex material for eight hours a day over an entire week, it’s hard for everyone to keep up,” he said. “With an on-demand and self-paced format, learners can review smaller modules daily, take their time to understand each topic, and repeat sections as often as they need.”
The 2026 Lead Auditor Training updates auditing practices for medical devices to include software. “Almost half of the new products coming to market today are software-based,” Packard said. “Yet, most auditor training programs don’t adequately cover how to evaluate software. This course fills that gap by addressing how to audit the software development process.”
Medical Device Academy’s global reach also makes the new course accessible to learners around the world. “More than half of our clients are located outside the U.S.,” Packard said. “By offering this training online, professionals anywhere can now gain the same level of instruction without needing to travel.”
In addition to individual learners, the course can be used for corporate training programs. “Companies can choose to train their entire auditing teams, with each employee receiving their own account,” Packard said. “That way, everyone can track their progress, access updated materials, and maintain training records in one place.”
Packard emphasized that course participants will receive ongoing updates as part of the program. “One of the things we do differently is that all our courses include updates at no additional cost,” he said. “When new standards or technologies are introduced, users automatically get access to new modules and can refresh their certification with the latest information.”
The course also introduces a multimedia learning experience, combining video instruction with real-world examples and storytelling for a more engaging format. “In most auditor training, you might face endless slides filled with text,” Packard said. “We wanted to move away from that. Our course features an instructor presenting on camera alongside visuals that reinforce key concepts, creating a more dynamic and effective learning experience.”
Packard, who has delivered auditor training across multiple continents, sees the move to an on-demand, self-paced model as a natural evolution. “Everywhere I have taught, from Europe to Asia to the U.S., the biggest challenge has been scheduling and learning retention,” he said. “This approach aims to solve both problems. Learners can start when they are ready and revisit any topic as often as they need. The course also includes pop quizzes to verify you understood the concepts.”
With its December launch approaching, Medical Device Academy is releasing new videos and blog content weekly as a sneak preview of the course.
Media Contact
Name: Rob Packard
Email: [email protected]
Original Source of the original story >> Medical Device Academy Announces Auditor Training for SaMD




