Autotelic Lab, Fountain Valley, a developer of quantitative rapid tests, announced today that it will be presenting a poster "The Hormones BNP and FSH in Ovarian Cancer: Potential as Diagnostic Biomarkers" at ENDO 2012. Ovarian cancer accounts for approximately 3 percent of all cancers in women and is the fifth leading cause of cancer-related death among women in the United States. Ovarian cancer has the highest mortality of all cancers of the female reproductive system. This reflects, in part, a lack of early symptoms and effective ovarian cancer screening tests. Thus, ovarian cancer is often diagnosed at an advanced stage, after the cancer has spread beyond the ovary. Cancer Antigen 125 (CA125, aka Mucin16) is overproduced by ovarian cancer cells; however, it cannot be used as a diagnostic biomarker for ovarian cancer as it can be absent when disease is present, or levels can be high when no disease or no malignant disease exists. Serial changes in CA125 levels, if elevated, however, can be useful in assessment of disease status and progression. Ovarian epithelial cancer is more common in individuals with elevated Gonadotropin-releasing hormones (GnRH) including Follicle Stimulating Hormone (FSH) and Luteinizing Hormone (LH) such as postmenopausal women or women who have received treatment to induce ovulation. Conversely, reduced risk of ovarian cancer is associated with a history of multiple pregnancies, breastfeeding, oral contraceptive use, and estrogen replacement therapy, all of which are related to lower levels of and reduced exposure to FSH and LH. FSH regulates gene expression in ovarian tumors and causes neovascularization of ovarian cancers by increasing Vascular Endothelial Growth Factor (VEGF) expression through upregulation of survivin. We therefore examined FSH levels in relation to CA125 as potential diagnostic marker for ovarian cancer using a quantitative point-of-care device for FSH that was recently developed for therapeutic drug monitoring. The surprising potential of FSH and/or Brain Natriuretic Peptide (BNP) as diagnostic biomarkers for ovarian cancer will be presented.
Here, Dr. Larn Hwang and colleagues describe the development of rapid point-of-care tests for detection of these hormones that are quantitative and applicable to plasma, serum, as well as urine. As rapid point-of-care tests, these tests would allow for more effective screening for ovarian cancer. These findings will be presented at The Endocrine Society’s 94th Annual Meeting & Expo (ENDO), June 23-26, 2012, Houston, TX.
Abstract # 851968: The Hormones BNP and FSH in Ovarian Cancer. Larn Hwang, Kouros Motamed, Chao Hsiao, and Vuong Trieu. This poster has been scheduled for presentation on Monday, June 25, 2012. POSTER SESSION: Biomarkers, Metastasis & Tumor Progression, & Tumorigenesis - II (1:30 PM-3:30 PM). Presentation Time: 1:30 pm. Room: Expo
About ENDO 2012:
The Endocrine Society's 94th Annual Meeting & EXPO takes place in Houston, TX on June 23-26, 2012. ENDO 2012 is a unique opportunity to learn about the latest research in fields as diverse as obesity, endocrine disruptors, diabetes, growth hormones, sex steroids, thyroid cancer and much more. Held at the George R. Brown Convention Center, more than 7,500 scientists and clinicians from all over the world will assemble for this global and premier meeting of hormone research, health science and endocrinology.
About Autotelic Lab:
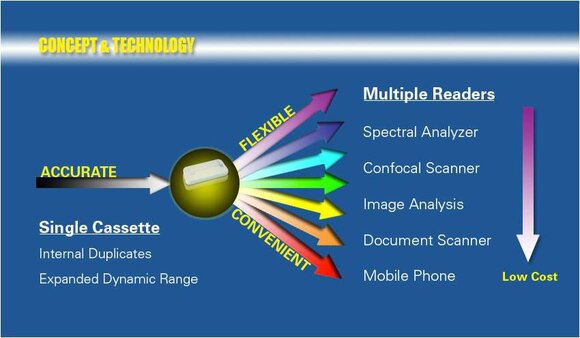
Autotelic lab is a provider of quantitative point-of-care testing solutions including quantitative lateral flow cassettes, lateral flow reader, as well as a quantification service. Our product lines include emergency care, women's health, cancer biomarkers, and regenerative medicine. Our offerings are uniquely configured to accept multiple readers to satisfy all possible field conditions; from highly specialized requiring specificity and sensitivity to low tech without access to normal laboratory resources. Our mission is to put testing into the hands of primary care providers and patients. These tests are simple to use and require no specialized equipment or trained personnel. They are rapid with results within 20 minutes of blood collection. They are convenient and can be deployed at home, in doctor’s office, in emergency rooms, or in centralized laboratories. Moreover, they are quantitative with a linear response range similar to tests performed at centralized laboratories.
These proprietary diagnostic devices deployed at point-of-care are being used for therapeutic drug monitoring by our partner?Biomiga Diagnostics? to personalize dosing of wide range of therapeutics e.g., chemotherapy and follicle hormone therapy (http://www.biomigadiagnostics.com).
The executives of Autotelic Lab are a group of pharmaceutical veterans who believe that the next revolution in medicine is in point-of-care tests for optimal drug dosing. With more than a decade of drug development experience, the leadership team has expertise ranging from basic/applied research, preclinical/clinical development, pharmacokinetics/pharmacodynamics biomarker development, mobile technology, regulatory, legal, marketing and business development. http://www.autoteliclab.com
Kouros Motamed, Ph.D.
Chief Executive Officer
[email protected]
- Home
- FSH and BNP as potential diagnostic biomarker for ovarian cancer
FSH and BNP as potential diagnostic biomarker for ovarian cancer
Last updated Monday, June 18, 2012 19:16 ETAutotelic Lab announced today that it will be presenting a poster The Hormones BNP and FSH in Ovarian Cancer: Potential as Diagnostic Biomarkers at ENDO 2012
Fountain Valley, 92708, 06/18/2012 / SubmitMyPR /








