The European Society of Hypertension-International Society of Hypertension hosted a Joint Conference virtually this year amid the Global Pandemic of COVID-19. The title of this virtual joint international conference was 'ESH-ISH HYPERTENSION 2021', and it was held from April 11-14, 2021, with live broadcast worldwide. Over 5,000 professionals in hypertension and related fields from around the world participated in this conference, and it focused primarily on implementing the Call to Action for Global Hypertension Control.
The President of the World Hypertension League, Professor Xin-Hua Zhang, delivered a keynote address on "Announcing and Promoting of the Call to Action for Global Hypertension Control". The World Hypertension League along with its global partners and member organizations drafted this speech.
According to the World Hypertension Congress that was held in São Paulo, Brazil, in 2019, despite being controllable, hypertension is not yet being properly controlled, leading risk factor for death and disability worldwide. There are more than 1.4 billion hypertensive patients around the world, but only less than 14% have their blood pressure under control.
The ESH-ISH HYPERTENSION 2021 called on global health authorities and medical professional societies to implement the following practices:
- Apply global best practices
- Help primary care services become central for hypertension prevention and control
- Promote teamwork among medical and non-medical staff to create patient-centered care.
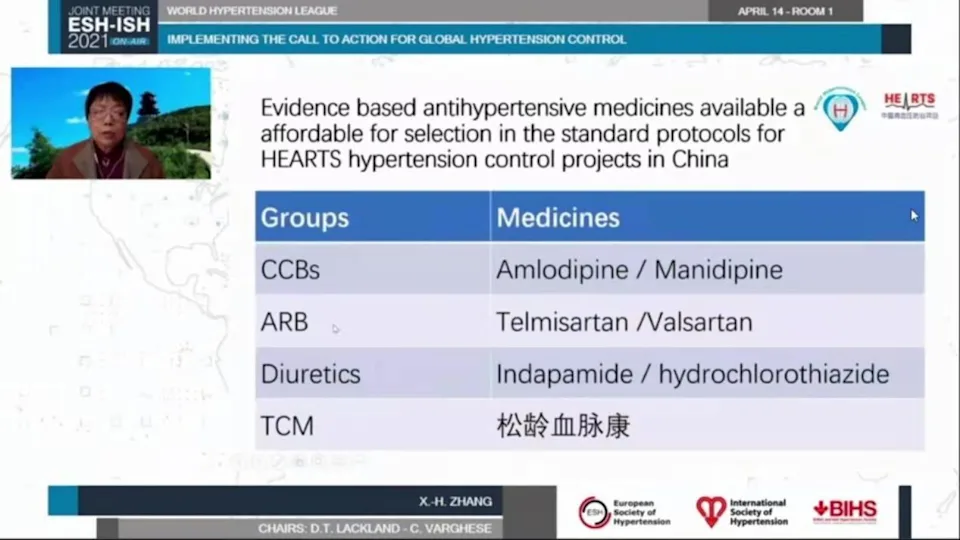
- Use evidence-based standardized treatment protocol, and continuous supply of antihypertensive medicines that are of good quality, inexpensive, and long-lasting.
- Patients should be identified by regular outpatient clinics and population screening services, and blood pressure should be controlled promptly.
- Excellent leaders, services, and groups committed to hypertension prevention and control efforts should be recognized.
Professor Xin-Hua Zhang reported on the HEARTS Hypertension Control Project in China's Henan Province. The effect of introducing the "Call to Action on Global Hypertension Control" has been well shown. The initiative is being led by the government's health officials, with the help of an advisory group, and is being conducted by healthcare providers in about 1000 township hospitals and community health centers. The project employs globally validated blood pressure, glucose, uric acid, and lipid measuring instruments, as well as web-based clinical support and a quality monitoring system. The system connects the measurements to the prescription platform, provides information to support clinicians' decision-making on diagnosis, treatment, and planning for follow-up. It also offers regular and weekly assessments to advise regional government offices about the effectiveness and advancement of hypertension management in the regions, allowing health officials to provide timely assistance and oversight to primary health care providers. The HEARTS hypertension control initiative was introduced in July 2018, and as of December 31, 2020, the number of primary care providers involved in the project has grown from 54 to over 800, with more than 40,000 registered hypertensive patients having their blood pressure under control.
The World Health Organization and international medical specialist societies created the HEARTS technical kit, which provides strategic and practical advice. Its goal is to enhance primary care services' ability to monitor and avoid cardiovascular diseases. Government health authorities, professional teams, and healthcare providers are also advised to use the kit. It provides information on safe habits and patient self-management, an evidence-based regulated treatment framework, high-quality, continuous delivery of medications and critical medical equipment in the chosen algorithm, risk-based management plans, coordination and mutual responsibility, and a real-time quality tracking process. The HEARTS professional kit is recommended as a standard procedure in the "Call to Action for Hypertension Control."
Traditional Chinese medications with RCT proof of successfully lowering blood pressure should play a significant role in hypertension management in medical care. In primary care settings, using an evidence-based standardized treatment algorithm is the most effective way to improve hypertension control. Evidence-based, long-acting, high-quality, inexpensive, accessible, and effective medications must be used in the algorithm. Scientific data should also be used to endorse the dosage for each phase and the evaluation pace in the algorithm. Chinese patent conventional medications that follow the above guidelines can be chosen in addition to internationally recommended antihypertensive drugs. "Songling Xuemaikang," for example, has RCT proof of successfully lowering blood pressure and improving the lipid profile. It is appropriate for the majority of patients who are at high risk of cardiovascular disease.
"The results of the HEARTS hypertension control project in Henan, China, demonstrate that the 'Call to Action' and its recommended best practices are implementable, replicable, and sustainable," Professor Zhang concluded. "Widely dissemination of Call to Action can help improve the global hypertension control and help more hypertensive patients live longer and healthier."
The European Society of Hypertension - +39 06 330 531